14+ What Are The Characteristics Of Metalloids On The Periodic Table Ideas
What are the characteristics of metalloids on the periodic table. The orange color on the Periodic table represents metalloids. Location on the Periodic Table The metalloids or semimetals are located along the line between the metals and nonmetals in the periodic table. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. A metalloid is a chemical element that exhibits some properties of metalsand some of nonmetals. All of the metalloids have some properties of both metals and. The metalloids separate the metals and nonmetals on a periodic table. The word metalloids is derived from the Latin word metallum meaning metal and the Greek word oeides meaning resembling in form or appearance. Boron B silicon Si germanium Ge arsenic As antimony Sb tellurium Te polonium Po and astatine At are the elements found along the step like line between metals and non-metals of the periodic table. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or. And these have intermediate properties with respect to metals and non-metals. They have properties of both metals and non-metals in the periodic table. On the periodic table those elements on the far left.
The elements that are pea-green in colour boron silicon germanium arsenic etc. Metalloids have semiconductor properties and form amphoteric oxides. In the periodic table metalloids form a jagged zone dividing elements that have clear metallic properties from elements that have clear nonmetallic properties. Metalloids are located between the metals and nonmetals. What are the characteristics of metalloids on the periodic table The physical properties of metalloids are those that can be observed without any change to the metalloid itself. Metalloids usually look like metals but behave largely like nonmetals. But elements that exhibit both metallic-like and nonmetallic properties make another class of semimetals or metalloids. The elements on the periodic table can be grouped as metals nonmetals or metalloids. In other words metalloids semimetals are located on the right side of the post transition metals and on the left side of nonmetals see above image. They form a separating boundary between the metals and nonmetals. Hence they appear between the metals and non-metals on the periodic table in a stair-step or staircase pattern. Physically they are shiny brittle solids with intermediate to relatively good electrical conductivity and the electronic band structure of a semimetal or semiconductor. They may display certain properties of metal or non-metals when reacting with certain chemicals.
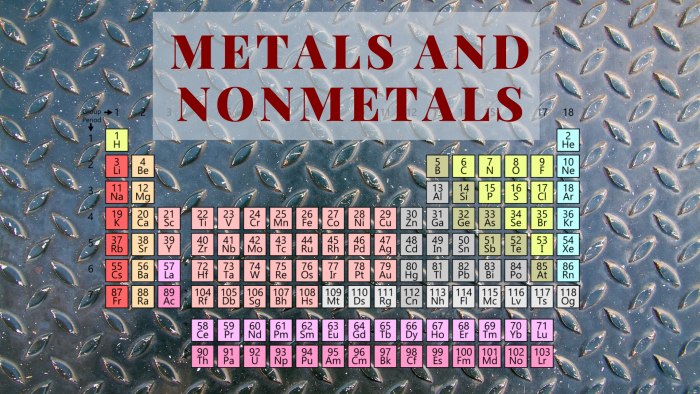 The Periodic Table Of Metals And Nonmetals Science Trends
The Periodic Table Of Metals And Nonmetals Science Trends
What are the characteristics of metalloids on the periodic table The line begins at boron B and extends down to polonium Po.
What are the characteristics of metalloids on the periodic table. Elements with properties intermediate between metalsand nonmetals. Metalloids exhibit the physical and chemical properties of both metals and non-metals on the periodic table. Elements to the left of the line are considered metals.
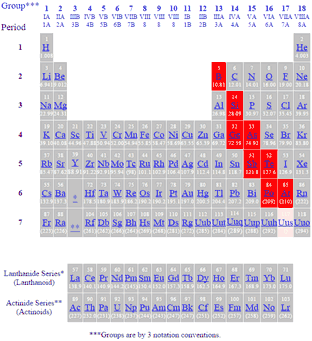
Metalloids have properties of both metals and non-metals. These elements are called metalloids or. Also many periodic tables have a stair-step line on the table identifying the element groups.
For easy classification of elements there are two main classes. The term metalloid comes from the Greek word metallon which means metal and edios meaning sort The metalloids are often seen forming amphoteric oxides and they behave as semiconductors. A periodic table has stairs like steps that help identify the element by groups.
What are the characteristics of metalloids on the periodic table A periodic table has stairs like steps that help identify the element by groups.
What are the characteristics of metalloids on the periodic table. The term metalloid comes from the Greek word metallon which means metal and edios meaning sort The metalloids are often seen forming amphoteric oxides and they behave as semiconductors. For easy classification of elements there are two main classes. Also many periodic tables have a stair-step line on the table identifying the element groups. These elements are called metalloids or. Metalloids have properties of both metals and non-metals. Elements to the left of the line are considered metals. Metalloids exhibit the physical and chemical properties of both metals and non-metals on the periodic table. Elements with properties intermediate between metalsand nonmetals.
What are the characteristics of metalloids on the periodic table
Indeed recently has been hunted by users around us, perhaps one of you personally. Individuals now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the name of this post I will talk about about What Are The Characteristics Of Metalloids On The Periodic Table.
What are the characteristics of metalloids on the periodic table. For easy classification of elements there are two main classes. The term metalloid comes from the Greek word metallon which means metal and edios meaning sort The metalloids are often seen forming amphoteric oxides and they behave as semiconductors. A periodic table has stairs like steps that help identify the element by groups. For easy classification of elements there are two main classes. The term metalloid comes from the Greek word metallon which means metal and edios meaning sort The metalloids are often seen forming amphoteric oxides and they behave as semiconductors. A periodic table has stairs like steps that help identify the element by groups.
If you are searching for What Are The Characteristics Of Metalloids On The Periodic Table you've come to the ideal place. We have 51 images about what are the characteristics of metalloids on the periodic table adding images, photos, photographs, backgrounds, and much more. In these webpage, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.